Scientists have understood for some time that the most abundant elements in the Universe are simple gases like hydrogen and helium. These make up the vast majority of its observable mass, dwarfing all the heavier elements combined (and by a wide margin). And between the two, helium is the second lightest and second most abundant [...]
Scientists have understood for some time that the most abundant elements in the Universe are simple gases like hydrogen and helium. These make up the vast majority of its observable mass, dwarfing all the heavier elements combined (and by a wide margin). And between the two, helium is the second lightest and second most abundant element, being present in about 24% of observable Universe’s elemental mass.
Whereas we tend to think of Helium as the hilarious gas that does strange things to your voice and allows balloons to float, it is actually a crucial part of our existence. In addition to being a key component of stars, helium is also a major constituent in gas giants. This is due in part to its very high nuclear binding energy, plus the fact that is produced by both nuclear fusion and radioactive decay. And yet, scientists have only been aware of its existence since the late 19th century.
Discovery and Naming:
The first evidence of helium was obtained on August 18th, 1868 by French astronomer Jules Janssen. While in Guntur, India, Janssen observed a solar eclipse through a prism, whereupon he noticed a bright yellow spectral line (at 587.49 nanometers) emanating from the chromosphere of the Sun. At the time, he believed it to be sodium, since it was proximate to the D1 and D2 Fraunhofer lines.
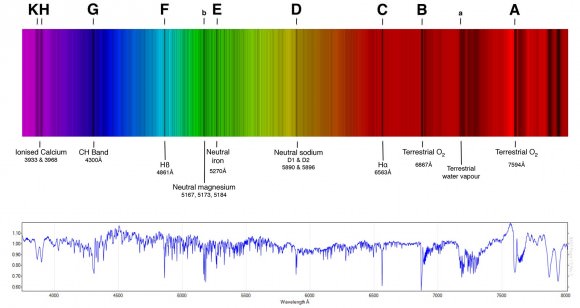
On October 20th of that same year, English astronomer Norman Lockyer observed a yellow line in the solar spectrum (which he named the D3 Fraunhofer line) which he concluded was caused by an unknown element in the Sun. Lockyer and English chemist Edward Frankland named the element helios, after the Greek word for the Sun.
Characteristics:
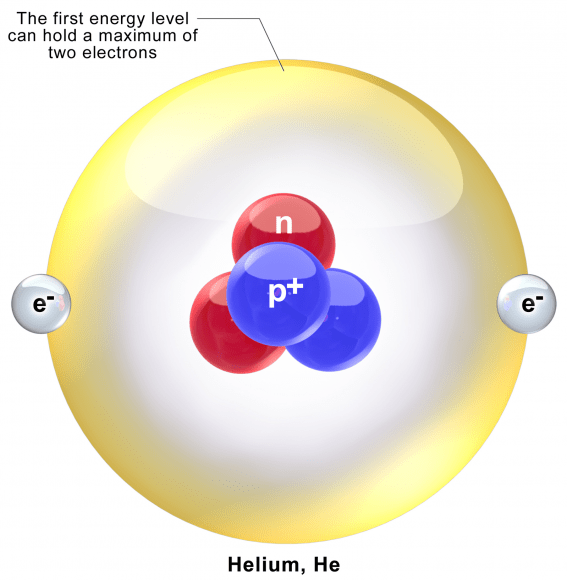
Helium is the second simplest atom when it comes to its atomic model, following hydrogen. It consists of a nucleus of two protons and neutrons, and two electrons in atomic orbits. The most common form is Helium-4, which is believed to be the product of Big Bang nucleosynthesis. This event, which lasted from 10 seconds to 20 minutes after the Big Bang, was characterized by the production of nuclei other than the lightest isotope of hydrogen (i.e. hydrogen-1. which has a single proton and nucleus).
This event is believed to have produced the majority of helium-4, along with small amounts of the hydrogen, helium and lithium isotopes. All other heavier elements were created much later, as a result of stellar nucleosynthesis. Large amounts of new helium are being created all the time through this same process, where the heat and pressure at the core of stars are causing hydrogen atoms to fuse.
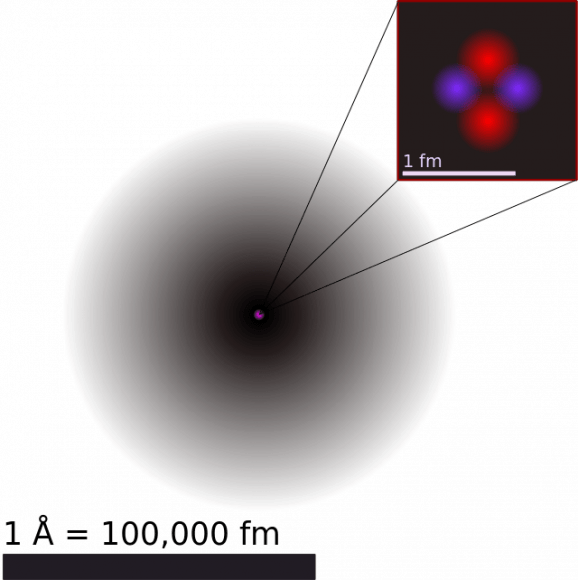
The nucleus of the helium-4 atom is identical with an alpha particle, two bound protons and neutrons that are produced in the process of alpha decay (where an element decays, releasing mass and becoming something else). The inertness of helium is due to the stability and low energy of it’s electron cloud state, where all of its electrons fully occupy 1s orbitals in pairs, none possessing angular momentum and each cancels the other’s intrinsic spin.
This stability also accounts for the lack of interaction of helium atoms with each other, which leads to one of he lowest melting and boiling points of all the elements.
History of Use:
For some time, helium was believed to exist only in the Sun. However, in 1882, Italian physicist Luigi Palmieri detected helium on Earth when analyzing lava from Mount Vesuvius after it erupted in that year. And in 1895, while searching for argon, Scottish chemist Sir William Ramsay managed to isolate helium by treating a sample of cleveite with mineral acids. After treating the element with sulfuric acid, he noticed the same D3 absorption line.
Ramsey sent samples of the gas to Sir William Crookes and Sir Norman Lockyer, who verified that it was helium. It was independently isolated from cleveite the same year by chemists Per Teodor Cleve and Abraham Langlet in Uppsala, Sweden, who were able to accurately determine its atomic weight. Over the course of the next few years, similar experiments yielded the same results.
Several interesting properties of helium were discovered in the ensuing years. In 1907, Ernest Rutherford and Thomas Royds demonstrated that an alpha particle is actually a helium nucleus. In 1908, helium was first liquefied by Dutch physicist Heike Kamerlingh Onnes by cooling the gas to less than one kelvin. The element was eventually solidified in 1926 by his student Willem Hendrik Keesom, who subjected the element to 25 atmospheres of pressure.
Helium was one of the first elements to be found to have superfluidity. In 1938, Russian physicist Pyotr Leonidovich Kapitsa discovered that helium-4 has almost no viscosity at temperatures near absolute zero (superfluidity). In 1972, the same phenomenon was observed in helium-3 by American physicists Douglas D. Osheroff, David M. Lee, and Robert C. Richardson.
Modern Uses:
Today, helium gas is used in a wide range of industrial, commercial and recreational applications. The most well-known is perhaps flight, where helium gas (being lighter than air) naturally provides buoyancy for airships and balloons. Compared to hydrogen, which was also used in airships, helium has the added benefit of being non-flammable and fire retardant.
Owing to its unique properties – which include a low boiling point, low density, low solubility, high thermal conductivity and inertness – helium is used for a wide range of scientific and medical applications. The greatest use is in cryogenic applications, where liquid-helium acts as a coolant for superconducting magnets in MRI scanners and spectrometers.

Another use is in rocketry, where helium is used as a buffer to displace fuel and oxidizers in storage tanks. It is also used to condense hydrogen and oxygen into rocket fuel and pre-cool liquid hydrogen in space vehicles. The Large Hadron Collider at CERN also relies on liquid helium to maintain a constant temperature of 1.9 kelvin.
Thanks to its extremely low index of refraction and the way it reduces the distorting effects of temperature variation, helium is also used in solar telescopes, gas chromatography, and in “helium dating” – i.e. determining the age of rocks that contain radioactive substances (like uranium and thorium). In addition to its inertness, its thermal properties, high speed of sound, and the high value of the heat capacity ration, it is also used in supersonic wind tunnels and aerodynamic testing facilities. It is also used in arc welding and for industrial leak detection.
We have written many interesting articles related to Helium here at Universe Today. Here’s Merging White Dwarfs Create Helium Stars, and Could Jupiter and Saturn Contain Liquid Metal Helium.
Astronomy Cast also has a good episode on the subject – Episode 139: Energy Levels and Spectra.


COMMENTS